Valsartan is an angiotensin II receptor antagonist primarily used to treat high blood pressure and heart failure. Its role in reducing cardiovascular risks makes it a widely prescribed medication globally. Understanding the production cost of Valsartan is essential for pharmaceutical manufacturers and healthcare providers, as it directly impacts pricing, market competitiveness, and availability. The production cost of Valsartan depends on various factors such as raw material costs, manufacturing processes, and overall efficiency of production.
This blog post delves into the key aspects of the Valsartan production cost, outlining its production process, raw material requirements, and the associated costs. It will also explore Valsartan’s chemical structure, its solubility, and potential side effects, along with recent news impacting the Valsartan market.
Production Process
The production of Valsartan involves several intricate steps, requiring precision to ensure the final product meets pharmaceutical-grade quality standards. Valsartan synthesis typically starts with the condensation of 4’-bromomethylbiphenyl-2-carboxylic acid with a tetrazole derivative. Following this, the chemical process involves cyclization reactions that lead to the formation of the angiotensin II receptor antagonist.
Request For Sample: https://www.procurementresource.com/production-cost-report-store/valsartan/request-sample
The synthesis requires careful control of reaction conditions to optimize yield while maintaining the stability of the Valsartan molecule. Chemical reagents, solvents, and catalysts play a crucial role in the production process, and minimizing by-products is essential to ensure the final product is free of impurities. The production process can be optimized by adopting efficient reaction pathways and using advanced production technologies, such as continuous flow chemistry, to reduce operational costs.
Manufacturing Report and Process
The manufacturing of Valsartan, on an industrial scale, follows Good Manufacturing Practices (GMP) to meet regulatory standards set by pharmaceutical authorities. Key components of the manufacturing process include:
- Synthesis of Active Pharmaceutical Ingredient (API): As previously mentioned, Valsartan synthesis involves complex chemical reactions that produce the API. The quality of the final API batch depends on the purity of raw materials and the precision of each chemical step.
- Purification: Once the API is synthesized, it undergoes a purification process, usually through crystallization, filtration, or chromatography. Purity is critical to ensure the therapeutic efficacy of the drug and to minimize side effects.
- Formulation: After purification, Valsartan is combined with excipients to create its final dosage form, typically in tablet or capsule form. The excipients act as fillers or stabilizers and play a role in determining the drug’s bioavailability and shelf life.
- Packaging and Quality Control: The final stage involves packaging the drug in tamper-evident containers, accompanied by the required labeling and safety warnings. Each batch undergoes rigorous quality control checks to ensure consistency, purity, and effectiveness.
Cost optimization in the manufacturing process often revolves around enhancing the efficiency of chemical reactions, reducing waste, and improving yield during purification. Any advancements in this area can help pharmaceutical companies reduce production costs, translating into more affordable pricing for consumers.
Raw Material Costs
The production of Valsartan requires several critical raw materials, the costs of which significantly influence the overall production expenses. The primary raw materials include:
- Chemical Reagents: Reagents such as biphenyl derivatives and tetrazole derivatives are essential for the synthesis of Valsartan. Prices of these chemicals fluctuate based on global supply chains, availability of raw materials, and market demand.
- Solvents and Catalysts: Various solvents (e.g., ethanol, acetonitrile) and catalysts are used to optimize the chemical reactions in Valsartan production. The choice of solvent affects not only the cost but also the yield and environmental impact of the production process.
- Excipients: After synthesizing Valsartan, excipients such as microcrystalline cellulose, magnesium stearate, and lactose are added during the formulation stage to ensure proper drug delivery. The costs of these excipients are typically stable but may vary based on supplier contracts and market conditions.
- Packaging Materials: The cost of packaging materials, including blister packs, bottles, and labeling, also factors into the overall production cost. Packaging costs are influenced by material availability, sustainability initiatives, and market regulations.
Pharmaceutical companies typically seek to minimize raw material costs by engaging in long-term supplier contracts, strategic sourcing, and bulk purchasing.
Valsartan Structure, Valsartan Side Effects, Valsartan Classification, Valsartan Solubility, Valsartan Contraindications
- Valsartan Structure:
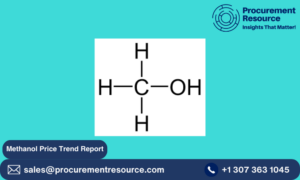
Valsartan is a biphenyl-tetrazole derivative. Its chemical structure is C24H29N5O3, featuring a carboxyl group, tetrazole ring, and biphenyl backbone. This unique structure allows Valsartan to block the angiotensin II receptor, reducing the blood vessel constriction and lowering blood pressure. - Valsartan Side Effects:
While Valsartan is effective in treating hypertension and heart failure, it can cause certain side effects. The most common side effects include dizziness, fatigue, headaches, and low blood pressure. Serious side effects are rare but may include kidney problems, increased potassium levels, and allergic reactions. - Valsartan Classification:
Valsartan belongs to the class of drugs known as angiotensin II receptor blockers (ARBs). These medications work by preventing angiotensin II from binding to its receptors, thereby preventing the narrowing of blood vessels and aiding in blood pressure control. - Valsartan Solubility:
Valsartan is classified as practically insoluble in water, but it is soluble in organic solvents such as ethanol. This solubility profile plays a significant role in the drug’s formulation, as it determines how the medication is absorbed in the body. - Valsartan Contraindications:
Valsartan is contraindicated in patients with hypersensitivity to the drug, those with severe hepatic or renal impairment, and pregnant women, as it can cause harm to the fetus. Patients taking Valsartan should also avoid using potassium supplements or salt substitutes, as it can lead to hyperkalemia (high potassium levels).
Latest News
In recent years, the Valsartan market has faced challenges due to contamination issues. In 2018, several batches of Valsartan were found to contain impurities such as N-nitrosodimethylamine (NDMA), a substance classified as a probable human carcinogen. This discovery led to widespread recalls of Valsartan products across multiple regions, creating supply shortages and driving up prices.
Since then, regulatory bodies like the FDA and the European Medicines Agency (EMA) have imposed stricter manufacturing guidelines to prevent further contamination. The pharmaceutical industry has been under pressure to implement more stringent quality control measures and improve the traceability of raw materials.
In response to these challenges, some manufacturers have adopted advanced production technologies to reduce contamination risks and improve efficiency. Continuous flow chemistry, for example, is being explored as a method to increase the safety and consistency of Valsartan production.
Furthermore, with the rising demand for affordable hypertension treatments, there is growing interest in developing generic versions of Valsartan. Generic manufacturers are focusing on optimizing production costs while maintaining high-quality standards to meet the increasing global demand.
Contact Us:
Company Name: Procurement Resource
Contact Person: Endru Smith
Email: sales@procurementresource.com
Toll-Free Number: USA & Canada - Phone no: +1 307 363 1045 | UK - Phone no: +44 7537 132103 | Asia-Pacific (APAC) - Phone no: +91 1203185500
Address: 30 North Gould Street, Sheridan, WY 82801, USA
Website: https://www.procurementresource.com/