Isorhamnetin is a naturally occurring flavonoid, primarily found in various fruits, vegetables, and medicinal plants. With its powerful antioxidant and anti-inflammatory properties, isorhamnetin has gained considerable attention in the fields of nutraceuticals and pharmaceuticals. This blog delves into the production process of isorhamnetin, its benefits, and its chemical structure.
What is Isorhamnetin?
Isorhamnetin is a flavonoid that belongs to the subclass of flavonols. Its presence in plants is often linked to their bright coloration, and it is typically found in sources like sea buckthorn, ginkgo biloba, onions, and green tea. This compound is commonly known for its various health benefits, which makes it a valuable ingredient in dietary supplements and herbal products.
Isorhamnetin Production Process
The production process of isorhamnetin involves several key steps, often beginning with the extraction from plant sources and purification to achieve the desired compound concentration. Here is a step-by-step breakdown of the general production process:
Request For Sample: https://www.procurementresource.com/production-cost-report-store/isorhamnetin/request-sample
1. Raw Material Selection
- The primary step involves selecting high-quality plant sources rich in isorhamnetin, such as sea buckthorn berries and ginkgo biloba leaves.
- Once collected, the plant material undergoes drying and grinding to facilitate the extraction process.
2. Extraction
- Solvent Extraction: The ground plant material is treated with a suitable solvent, commonly methanol or ethanol, to dissolve isorhamnetin.
- Ultrasonic-Assisted Extraction (UAE): This modern technique enhances the efficiency of extraction. Ultrasonic waves disrupt the cell walls, releasing more isorhamnetin into the solvent.
- Supercritical Fluid Extraction: Carbon dioxide under supercritical conditions acts as an excellent solvent, selectively extracting isorhamnetin without leaving any solvent residue.
3. Filtration and Separation
- The extract is filtered to remove plant debris and impurities. The solution may then undergo centrifugation to further separate fine particles and other unwanted materials.
- Liquid-Liquid Extraction is also used to partition isorhamnetin into an organic solvent, while other compounds remain in the aqueous phase.
4. Purification and Concentration
- Column Chromatography: To obtain a pure form of isorhamnetin, column chromatography is widely used. Different types of chromatographic techniques, like high-performance liquid chromatography (HPLC), are essential in isolating high-purity isorhamnetin.
- Vacuum Concentration: The purified isorhamnetin solution is concentrated by evaporating excess solvent under reduced pressure, yielding a concentrated form of isorhamnetin.
5. Crystallization and Drying
- Crystallization: In this step, the concentrated solution is cooled, encouraging the formation of isorhamnetin crystals. The crystals are then collected through filtration.
- Drying: The final step involves drying the crystallized isorhamnetin under controlled conditions, ensuring the stability of the compound. The dried isorhamnetin is then prepared for packaging and storage.
Benefits of Isorhamnetin
Isorhamnetin is associated with several health benefits, making it a sought-after compound in the nutraceutical industry. Here are some of its most notable benefits:
1. Antioxidant Properties
- Isorhamnetin acts as a potent antioxidant, neutralizing free radicals and protecting cells from oxidative damage. This function is crucial in preventing age-related diseases and supporting overall health.
2. Anti-Inflammatory Effects
- The compound has demonstrated anti-inflammatory effects, helping reduce inflammation in various tissues. This benefit is particularly valuable in managing conditions like arthritis, where inflammation plays a key role.
3. Cardiovascular Health
- Studies have shown that isorhamnetin may help improve heart health by reducing cholesterol levels, enhancing blood circulation, and preventing the oxidation of low-density lipoprotein (LDL), which can lead to atherosclerosis.
4. Anti-Cancer Potential
- Preliminary research suggests that isorhamnetin may inhibit the growth of certain cancer cells, particularly in breast and prostate cancers. While more studies are needed, this potential has generated considerable interest in oncology.
5. Supports Brain Health
- Due to its neuroprotective properties, isorhamnetin has been linked to better cognitive health and may help reduce the risk of neurodegenerative disorders like Alzheimer’s disease.
Structure of Isorhamnetin
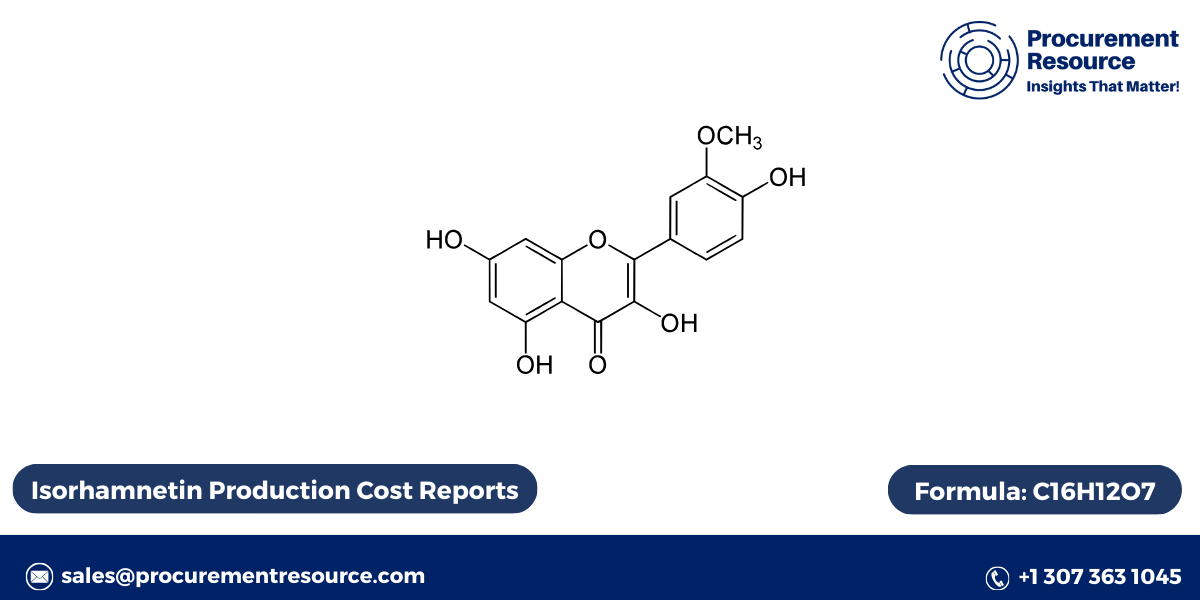
Isorhamnetin has a typical flavonoid structure, consisting of three rings (C6-C3-C6) connected by a three-carbon bridge. Its molecular formula is C16H12O7, and the compound features a methoxy group (-OCH3) attached to the B-ring. This methoxy group differentiates it from other closely related flavonoids, such as quercetin.
Chemical Structure:
Isorhamnetin has a structure that includes:
- Two benzene rings (A and B): These rings contain hydroxyl groups, which contribute to its antioxidant activity.
- A heterocyclic ring (C): The C-ring, also known as the pyran ring, connects the two benzene rings.
The hydroxyl and methoxy groups in the structure of isorhamnetin are critical for its biochemical activity, as they participate in free radical scavenging and interact with various biological molecules.
Isorhamnetin is a powerful flavonoid with a well-defined production process and numerous health benefits. Its natural antioxidant and anti-inflammatory properties make it a popular ingredient in dietary supplements and herbal remedies. Whether through solvent extraction or advanced chromatography, the production of isorhamnetin continues to evolve, aiming to yield higher purity and efficiency. As research advances, the potential applications of isorhamnetin in pharmaceuticals and nutraceuticals will likely continue to expand, offering new ways to harness its health-promoting properties.
Contact Us:
Company Name: Procurement Resource
Contact Person: Endru Smith
Email: sales@procurementresource.com
Toll-Free Number: USA & Canada - Phone no: +1 307 363 1045 | UK - Phone no: +44 7537 132103 | Asia-Pacific (APAC) - Phone no: +91 1203185500
Address: 30 North Gould Street, Sheridan, WY 82801, USA



