Introduction: Retapamulin Production Process with Cost Analysis
The Retapamulin Production Process is integral to the pharmaceutical industry, specifically for the development of topical antibiotics used to treat skin infections. As a semi-synthetic pleuromutilin antibiotic, retapamulin requires a specialized production process involving advanced chemical synthesis. This report provides a comprehensive overview of the retapamulin production process, including cost analysis, resource procurement, market drivers, and critical factors impacting production costs, offering valuable insights for stakeholders looking to optimize production or enter this niche market.
Request Free Sample – https://www.procurementresource.com/production-cost-report-store/retapamulin/request-sample
Procurement Resource Assessment: Retapamulin Production Process
An effective Procurement Resource Assessment is essential for optimizing the retapamulin production process. This assessment involves evaluating the availability, quality, and cost-effectiveness of essential resources, including raw materials, labor, equipment, and energy.
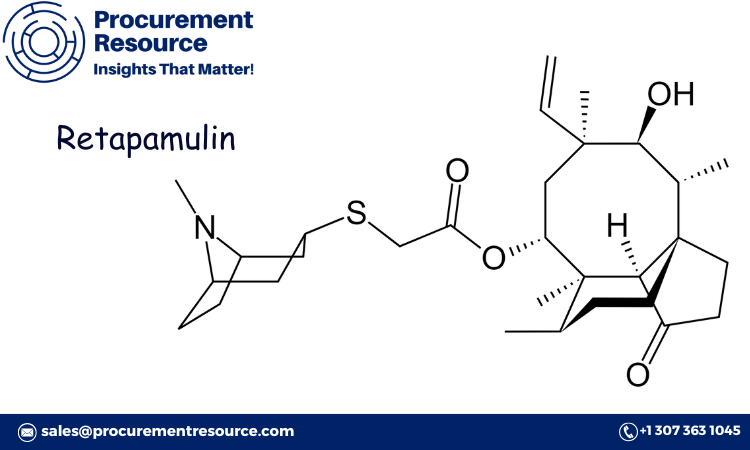
Retapamulin is produced through a semi-synthetic process that begins with pleuromutilin, a naturally occurring antibiotic compound isolated from certain fungi. The production process involves modifying pleuromutilin’s structure through chemical reactions to enhance its antibacterial efficacy and improve its stability. Key stages in the retapamulin production process include:
- Isolation of Pleuromutilin: The production begins with the isolation of pleuromutilin from fungal sources. In commercial-scale production, synthetic pleuromutilin analogs are often used to maintain consistency in supply and product quality.
- Chemical Modification: The isolated or synthesized pleuromutilin undergoes several chemical modification steps to produce retapamulin. These steps involve the use of catalysts, reagents, and solvents to alter the molecular structure while preserving the antibiotic properties of the compound.
- Purification and Crystallization: Following synthesis, the compound is purified through filtration, crystallization, and other separation techniques to remove impurities. These steps are essential to ensure that the final product meets pharmaceutical-grade quality standards.
- Formulation and Packaging: Once purified, retapamulin is formulated into ointments for topical application. Excipients are added to enhance stability and ease of application, and the final product is packaged under sterile conditions to ensure safety and efficacy.
A thorough procurement assessment helps companies identify reliable suppliers for high-quality raw materials, implement cost-efficient sourcing strategies, and establish a robust supply chain, all of which are essential for consistent and compliant production.
Retapamulin Overview
Retapamulin is a topical antibiotic in the pleuromutilin class, used primarily to treat skin infections caused by Gram-positive bacteria, such as impetigo. It inhibits bacterial protein synthesis by binding to the 50S ribosomal subunit, a unique mechanism of action that reduces the risk of cross-resistance with other antibiotics.
Approved for topical use in the form of ointments, retapamulin is particularly effective against resistant strains of bacteria like MRSA. Due to its unique structure and mechanism, retapamulin offers an alternative for patients who do not respond well to other topical antibiotics. The growing concern over antibiotic resistance has highlighted the importance of antibiotics like retapamulin, which provide effective treatment options with a low potential for resistance development.
Market Drivers
Several Market Drivers are influencing the demand for retapamulin:
- Rising Prevalence of Skin Infections: Skin infections are among the most common infections treated in outpatient settings, driving the demand for topical antibiotics like retapamulin. The increasing prevalence of conditions like impetigo, particularly in children, contributes to the steady demand for retapamulin-based treatments.
- Growing Concerns Over Antibiotic Resistance: Antibiotic resistance is a major public health challenge. Retapamulin’s unique mechanism of action provides an alternative to conventional antibiotics, making it an important option in the treatment of resistant bacterial infections.
- Expansion of the Dermatology and Skincare Markets: The expansion of dermatology and skincare markets has led to greater demand for treatments targeting bacterial skin infections. This trend is particularly notable in developing countries, where awareness of dermatological health is increasing.
- Increased Research and Development in Antibiotics: The pharmaceutical industry continues to invest in developing new antibiotics and improving existing ones. Retapamulin benefits from this trend, as its efficacy against drug-resistant bacteria has made it a focal point in antibiotic R&D.
- Government and Regulatory Support for Antibiotic Development: Many governments and regulatory agencies support the development of antibiotics through incentives and funding initiatives. This support is crucial in driving innovation in antibiotic development and contributes to the availability of effective treatments like retapamulin.
Raw Materials Requirements
The Raw Materials Requirements for retapamulin production depend on the specific production method used, with pleuromutilin as the primary raw material. Key raw materials include:
- Pleuromutilin: The primary starting material, pleuromutilin, is isolated from fungal sources or synthesized. High-purity pleuromutilin is essential for ensuring the efficacy and quality of the final product.
- Reagents and Catalysts: Chemical reagents and catalysts are used to modify pleuromutilin into retapamulin. Commonly used reagents include acids, bases, and various organic compounds. Catalysts facilitate reaction rates and yields.
- Solvents: Solvents, such as ethanol or methanol, are used throughout the synthesis and purification stages. Solvents help dissolve reagents, control reaction conditions, and assist in isolating the final product.
- Excipients: For formulation into topical ointments, additional excipients are required. These may include stabilizers, emollients, and preservatives that ensure the stability, bioavailability, and efficacy of the final product.
- Packaging Materials: Pharmaceutical-grade packaging materials, such as tubes or jars, are required to protect the ointment from environmental factors and ensure patient safety.
Costs and Key Process Information
Understanding the Costs and Key Process Information is essential for optimizing the retapamulin production process. Major cost components include raw materials, labor, equipment, energy, and regulatory compliance.
- Raw Material Costs: The cost of pleuromutilin and other raw materials, such as reagents and solvents, represents a significant portion of production expenses. Sourcing high-purity pleuromutilin from reliable suppliers is crucial to maintain product quality and cost-effectiveness.
- Labor Costs: Skilled labor is required for various stages of production, including chemical modification, purification, formulation, and quality control. Labor costs vary depending on the geographic location and scale of operations.
- Equipment and Facility Costs: Retapamulin production facilities require specialized equipment, such as reactors, crystallizers, and dryers, for synthesis and purification. Initial capital investments are substantial, particularly for large-scale production. Ongoing maintenance costs are necessary to ensure safety and operational efficiency.
- Energy Costs: The production process is energy-intensive, particularly during chemical synthesis and purification. Efficient energy management is critical to minimizing costs and reducing the environmental impact of production. Many producers are exploring renewable energy options to enhance sustainability.
- Environmental Compliance: Retapamulin production is subject to strict environmental and pharmaceutical regulations. Compliance requires investments in emissions control, waste management, and quality control systems, all of which contribute to overall production costs.
Looking for an Exhaustive and Personalized Report that Could Significantly Substantiate Your Business
For those seeking a comprehensive understanding of the retapamulin production process, a Personalized Report provides tailored insights specific to your business needs. Such a report offers:
- A detailed breakdown of production costs and potential cost-saving measures.
- Recommendations for sourcing high-quality raw materials and establishing reliable supplier relationships.
- Market analysis, including trends, competitive landscape, and growth opportunities within the pharmaceutical industry.
- Guidance on optimizing energy use, waste management, and environmental sustainability.
- Insights into regulatory compliance and quality control measures to ensure adherence to industry standards and maintain product integrity.
An exhaustive report provides the critical information necessary to navigate the complexities of the retapamulin market, enabling businesses to make informed decisions, optimize production processes, and capitalize on growth opportunities in the pharmaceutical industry.
About Us:
Procurement Resource is an invaluable partner for businesses seeking comprehensive market research and strategic insights across a spectrum of industries. With a repository of over 500 chemicals, commodities, and utilities, updated regularly, they offer a cost-effective solution for diverse procurement needs. Their team of seasoned analysts conducts thorough research, delivering clients with up-to-date market reports, cost models, price analysis, and category insights.
By tracking prices and production costs across various goods and commodities, Procurement Resource ensures clients receive the latest and most reliable data. Collaborating with procurement teams across industries, they provide real-time facts and pioneering practices to streamline procurement processes and enable informed decision-making. Procurement Resource empowers clients to navigate complex supply chains, understand industry trends, and develop strategies for sustainable growth.
Contact Us:
Company Name: Procurement Resource
Contact Person: Amanda Williams
Email: sales@procurementresource.com
Toll-Free Number: USA Canada – Phone no: +1 307 363 1045 | UK – Phone no: +44 7537 132103 | Asia-Pacific (APAC) – Phone no: +91 1203185500
Address: 30 North Gould Street, Sheridan, WY 82801, USA



