L-Ornithine Production Process with Cost Analysis: A Comprehensive Guide for Business Optimization
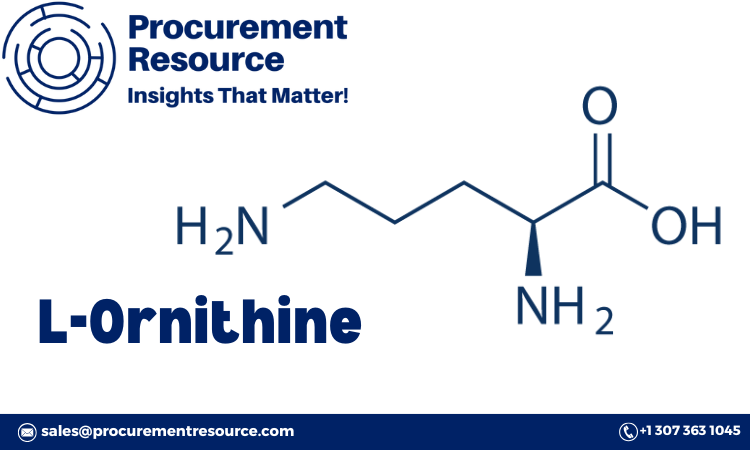
L-Ornithine, an amino acid with significant applications in health supplements, pharmaceuticals, and functional foods, is valued for its role in metabolic and detoxification processes. As demand for amino acids in wellness and medical applications grows, understanding the L-Ornithine Production Process with Cost Analysis is essential for businesses looking to optimize production and control costs effectively. This report provides a detailed overview of the L-Ornithine production process, including procurement resources, market drivers, raw material requirements, cost considerations, and how a personalized report can enhance business strategies.
Request For Free Sample: https://www.procurementresource.com/production-cost-report-store/l-ornithine/request-sample
1. Procurement Resource Assessment for L-Ornithine Production Process
The L-Ornithine Production Process requires the efficient sourcing of raw materials and specialized equipment to ensure high yield and quality. Here’s an overview of key procurement considerations:
- Source Materials for L-Ornithine Production: L-Ornithine is typically produced either by extracting it from plant and animal sources or by synthesizing it through microbial fermentation. Fermentation processes use specific bacteria strains (such as Escherichia coli) or yeast that produce L-Ornithine through fermentation in bioreactors. Obtaining high-quality microbial cultures or source materials is crucial to maintain consistent production output.
- Fermentation Media and Supplements: In microbial fermentation, growth media enriched with nutrients like glucose, nitrogen, and minerals are required to support microbial growth and L-Ornithine production. Selecting and procuring high-quality fermentation media ensures optimal growth conditions, which improves yield and product purity.
- Specialized Extraction and Purification Systems: The extraction and purification of L-Ornithine involve specific processes such as centrifugation, crystallization, and chromatography. Reliable equipment is needed to ensure that the L-Ornithine produced meets quality standards suitable for pharmaceutical or food applications. Investing in advanced purification systems reduces contamination risks and enhances product quality.
An effective procurement strategy focusing on sourcing quality microbial cultures, nutrient-rich media, and reliable extraction equipment supports efficient and cost-effective L-Ornithine production.
2. Trypsin
While Trypsin is not directly used in the production of L-Ornithine, it plays a crucial role in various biochemical and pharmaceutical applications. Here’s a brief overview of Trypsin and its broader role in industrial processes:
- Protein Digestion in Pharmaceutical and Biotechnological Applications: Trypsin, a protease enzyme, is widely used to break down proteins into peptides and amino acids, which is essential in cell culture processes and protein analysis. It aids in cellular processes, such as detaching cells during culture, allowing for optimal cell growth and expansion.
- Applications in the Food and Supplement Industries: Trypsin is used in the food industry to modify proteins, improve texture, and enhance flavors. Its ability to break down complex proteins into simpler forms has made it valuable in processing protein-rich foods and supplements, aiding in nutrient bioavailability.
Though Trypsin is not involved in the direct production of L-Ornithine, its applications across various industries underscore its importance in enzymatic reactions and highlight its contribution to efficient production processes in biotechnology and pharmaceuticals.
3. Market Drivers
The L-Ornithine market is influenced by several key drivers, particularly the demand for wellness products and advancements in biotechnology. The primary market drivers include:
- Increasing Demand for Health and Wellness Supplements: L-Ornithine is commonly used in dietary supplements for its benefits in muscle recovery, liver health, and immune support. With the rising focus on health and wellness, the demand for amino acids like L-Ornithine is growing, particularly in the nutraceutical industry.
- Expanding Applications in Pharmaceuticals: L-Ornithine has therapeutic applications in treating liver disorders, improving athletic performance, and managing metabolic conditions. With increasing awareness of its medical benefits, the pharmaceutical sector’s demand for high-quality L-Ornithine is expected to grow, fueling market expansion.
- Growing Popularity of Functional Foods: As consumers become more interested in functional foods that offer additional health benefits, L-Ornithine is being incorporated into food and beverage products. This trend aligns with consumer preferences for functional ingredients that support overall health, particularly in products targeting energy and recovery.
These market drivers reflect the expanding role of L-Ornithine in health, wellness, and medical applications, highlighting the need for scalable and efficient production methods to meet rising demand.
4. Raw Materials Requirements
The L-Ornithine Production Process depends on specific raw materials, particularly when using microbial fermentation. Here’s an overview of the primary raw materials needed:
- Microbial Cultures or Source Materials: L-Ornithine can be sourced from various microbial cultures, such as Escherichia coli or Corynebacterium glutamicum, which are engineered for optimal L-Ornithine production. Alternatively, it can be derived from natural sources like plant proteins. Choosing the right strain or source material is crucial to ensure high yield and quality.
- Nutrient-Rich Fermentation Media: When producing L-Ornithine through fermentation, growth media containing carbohydrates (glucose), nitrogen sources, minerals, and vitamins are required. These nutrients support microbial growth and enhance L-Ornithine production, making media selection and quality crucial for process efficiency.
- Purification Reagents and Buffer Solutions: After L-Ornithine is produced, it must be extracted and purified. Buffer solutions, acids, and other reagents are used to separate L-Ornithine from impurities and stabilize it for use in supplements or pharmaceuticals. Ensuring a steady supply of these reagents helps maintain consistent product quality.
Securing these raw materials, along with high-quality microbial strains and nutrient-rich media, is essential for successful and efficient L-Ornithine production.
5. Costs and Key Process Information
The costs associated with the L-Ornithine Production Process are influenced by several factors, including raw material expenses, energy consumption, and regulatory compliance. Here’s a breakdown of the main cost components and process details:
- Raw Material Costs: The cost of microbial cultures, fermentation media, and purification reagents represents a significant portion of production expenses. Choosing reliable suppliers and implementing cost-effective sourcing strategies helps businesses manage these costs effectively.
- Labor and Operational Costs: Skilled personnel are required to oversee fermentation, monitor microbial growth, and perform quality control during extraction and purification. Labor costs are influenced by production scale, with larger facilities requiring additional resources. Operational costs also include maintenance of fermentation and purification equipment.
- Energy and Equipment Costs: L-Ornithine production, particularly via fermentation, involves energy-intensive steps like maintaining incubation temperatures and controlling pH levels. Investing in energy-efficient equipment can help reduce long-term expenses. Bioreactors, centrifuges, and filtration systems also represent significant capital investments, especially in large-scale production facilities.
- Quality Control and Regulatory Compliance: Ensuring that L-Ornithine meets quality standards for use in supplements or pharmaceuticals requires rigorous quality control. Adherence to Good Manufacturing Practices (GMP) and regulatory requirements add to the overall production costs, particularly for pharmaceutical-grade L-Ornithine.
Understanding these cost components allows businesses to optimize production efficiency, reduce expenses, and ensure compliance with industry regulations, supporting sustainable and cost-effective L-Ornithine manufacturing.
6. Looking for an Exhaustive and Personalized Report That Could Significantly Substantiate Your Business
For companies aiming to succeed in the L-Ornithine market, an exhaustive and personalized report provides detailed insights into key aspects of the production process and market dynamics. A customized report offers several advantages, including:
- Comprehensive Market Analysis and Forecasts: Access in-depth data on L-Ornithine market trends, demand forecasts, and competitive landscape assessments. These insights help businesses align their strategies with industry developments and capitalize on emerging opportunities in the health, wellness, and pharmaceutical sectors.
- Cost Optimization and Efficiency Recommendations: A personalized report includes analysis on cost-saving measures, such as process improvements, energy-efficient equipment, and alternative sourcing strategies. These recommendations support profitability and operational efficiency.
- Guidance on Regulatory Compliance and Quality Assurance: Navigating regulatory requirements for supplement and pharmaceutical production is essential for success. A customized report provides information on industry standards, best practices, and compliance requirements, helping your business meet local and international guidelines.
- Strategic Business Recommendations: Tailored insights based on your production capabilities, target markets, and operational objectives offer actionable recommendations for growth and competitiveness. These strategic insights guide decision-making and enhance market positioning.
Investing in a comprehensive, customized report enables businesses to make data-driven decisions and optimize their operations in the L-Ornithine market. With a detailed understanding of the L-Ornithine Production Process with Cost Analysis, companies can position themselves for success in the expanding nutraceutical, pharmaceutical, and functional food markets. Whether focusing on production efficiency, ensuring regulatory compliance, or exploring new product applications, a personalized report can substantiate your business strategy and support long-term growth.
Contact Us:
Company Name: Procurement Resource
Contact Person: Benking sley
Email: sales@procurementresource.com
Toll Free Number: USA & Canada – Phone no: +1 307 363 1045 | UK – Phone no: +44 7537 132103 | Asia-Pacific (APAC) – Phone no: +91 1203185500
Address: 30 North Gould Street, Sheridan, WY 82801, USA



