Introduction
The Ceftazidime Production Process with Cost Analysis is vital for pharmaceutical companies involved in the manufacture of antibiotics for bacterial infections. Ceftazidime, a third-generation cephalosporin antibiotic, is widely used to treat severe infections caused by gram-negative bacteria, such as Pseudomonas aeruginosa. This report provides a detailed overview of the ceftazidime production process, covering procurement resources, market drivers, raw material requirements, and cost considerations essential for understanding the manufacturing dynamics of this valuable antibiotic.
Request Free Sample – https://www.procurementresource.com/production-cost-report-store/ceftazidime/request-sample
Procurement Resource Assessment: Ceftazidime Production Process
The Procurement Resource Assessment for ceftazidime production involves identifying reliable sources of high-purity raw materials, access to specialized synthesis technology, and stringent quality control standards. Ceftazidime is typically produced through a complex chemical synthesis process involving advanced intermediates, acylation reactions, and purification stages. Effective procurement strategies for ceftazidime production must consider factors such as material consistency, cost-effectiveness, and adherence to regulatory standards.
Key procurement considerations include:
- High-Purity Precursors and Intermediates: The production of ceftazidime requires specific intermediates, such as 7-aminocephalosporanic acid (7-ACA) and various acylating agents. These must be sourced from reputable suppliers to maintain quality and consistency.
- Synthesis and Purification Technology: The synthesis of ceftazidime involves multi-step chemical reactions, including acylation and esterification. This requires specialized equipment like reactors, chromatographic systems, and filtration units to achieve high purity and efficiency.
- Regulatory Compliance: As a pharmaceutical product, ceftazidime production must adhere to Good Manufacturing Practices (GMP) and comply with international regulatory standards. Procurement must include quality assurance protocols and documentation to ensure compliance with these regulations.
A comprehensive procurement assessment considers these factors to optimize production efficiency, manage costs, and adhere to regulatory requirements for successful ceftazidime manufacturing.
Ceftazidime Overview
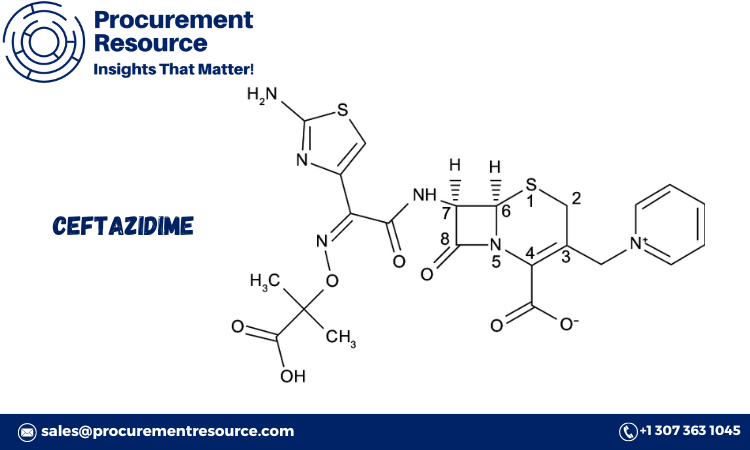
Ceftazidime is a third-generation cephalosporin antibiotic effective against a broad range of bacterial infections, particularly those caused by gram-negative bacteria. It works by inhibiting bacterial cell wall synthesis, leading to cell lysis and death. Due to its high efficacy and spectrum of activity, ceftazidime is commonly used to treat infections such as pneumonia, urinary tract infections, and sepsis, as well as infections in immunocompromised patients.
Ceftazidime’s broad-spectrum activity makes it a preferred choice for serious hospital-acquired infections and conditions requiring intravenous administration. Its role as a beta-lactam antibiotic means it is particularly effective against resistant strains, such as Pseudomonas aeruginosa, making it a critical component in modern infectious disease treatment.
Market Drivers
Several market drivers contribute to the demand for ceftazidime, including:
- Rising Prevalence of Bacterial Infections: The global incidence of infections, particularly hospital-acquired infections, has driven demand for effective antibiotics like ceftazidime. Its efficacy against drug-resistant bacteria is especially valuable in healthcare settings.
- Increased Focus on Antimicrobial Resistance: With growing concerns about antimicrobial resistance, there is an increasing need for advanced antibiotics. Ceftazidime’s effectiveness against gram-negative bacteria, including resistant strains, supports its role in combating resistant infections.
- Expansion of Healthcare Infrastructure: The growth of healthcare infrastructure, particularly in developing regions, has expanded access to antibiotics, supporting demand for medications like ceftazidime in treating severe infections.
- Advances in Pharmaceutical Research: Ongoing research into new applications for ceftazidime and other cephalosporins has increased its importance in the pharmaceutical industry. Its utility in treating complex and resistant infections contributes to its sustained market demand.
These market drivers highlight the importance of ceftazidime in modern infectious disease management, emphasizing its continued relevance in addressing global health challenges.
Raw Materials Requirements
The primary raw materials for ceftazidime production include chemical intermediates, acylating agents, and solvents for synthesis and purification. Below is an overview of the essential raw materials required at each stage of the production process:
- 7-Aminocephalosporanic Acid (7-ACA): This intermediate serves as the core building block for ceftazidime synthesis. Sourcing high-purity 7-ACA is essential to achieve the desired antibiotic efficacy and safety.
- Acylating Agents: Various acylating agents, such as pyridine derivatives, are used in the acylation step. These agents facilitate the attachment of functional groups to the 7-ACA core, forming the ceftazidime structure.
- Chemical Reagents and Solvents: Solvents like methanol, acetone, and dichloromethane are required in different synthesis stages. These solvents help control reaction conditions, aid in purification, and ensure efficient compound recovery.
- Purification Agents: High-performance liquid chromatography (HPLC) solvents, buffer solutions, and filtration aids are essential for the purification process. These materials are necessary to meet pharmaceutical-grade purity standards for ceftazidime.
The availability, cost, and quality of these raw materials significantly impact production efficiency, scalability, and compliance with pharmaceutical quality standards for ceftazidime.
Costs and Key Process Information
The costs associated with ceftazidime production depend on various factors, including raw material sourcing, production scale, and regulatory compliance. Here’s an overview of the key cost components and process steps:
1. Raw Material Costs:
- Intermediate and Acylating Agents: The cost of intermediates, such as 7-ACA, can fluctuate depending on market demand and supplier availability. Acylating agents and other reagents are also significant components of production expenses.
- Solvents and Reagents: The cost of solvents used throughout the production process varies based on volume requirements and solvent purity. Bulk purchasing helps reduce costs, but high-purity solvents are essential for pharmaceutical-grade production.
- Catalysts and Stabilizers: Certain catalysts and stabilizers may be required to facilitate reaction steps and ensure compound stability. These materials contribute to overall production costs and impact reaction efficiency.
2. Production and Processing Costs:
- Synthesis Reactions: Ceftazidime production involves multi-step chemical synthesis, requiring control over parameters like temperature, pH, and reaction time. This stage requires specialized equipment, such as reactors and temperature-controlled mixing units.
- Purification and Crystallization: Following synthesis, ceftazidime undergoes multiple purification steps to remove impurities and achieve pharmaceutical-grade purity. Filtration, crystallization, and chromatography techniques are used to ensure product quality.
- Quality Control and Product Testing: Rigorous quality control measures are necessary to ensure ceftazidime meets industry standards for safety, efficacy, and purity. Testing methods include HPLC analysis, microbial testing, and stability assays.
3. Operational and Compliance Costs:
- Labor and Expertise: Skilled chemists, laboratory technicians, and quality control specialists are essential for overseeing the production process. Labor costs vary depending on the facility location and the level of expertise required.
- Regulatory Compliance and Documentation: Ceftazidime production is subject to stringent regulatory requirements, including adherence to GMP and other pharmaceutical standards. Compliance costs include facility inspections, documentation, and regulatory submissions.
- Equipment and Facility Maintenance: The production of ceftazidime requires specialized equipment, including reactors, filtration systems, and chromatography columns. Regular maintenance ensures operational efficiency, minimizes downtime, and prolongs equipment life.
Looking for an Exhaustive and Personalized Report?
Are you interested in a comprehensive report tailored to meet your business’s specific needs in the ceftazidime production market? An exhaustive report on the Ceftazidime Production Process with Cost Analysis can provide valuable insights into raw material sourcing, production optimization, and cost management strategies. A customized report offers in-depth market intelligence and actionable recommendations to support your business’s competitive strategy and ensure regulatory compliance.
Our team of experts can develop a personalized report that addresses your unique business requirements, providing data-driven insights and strategic guidance.
About Us:
Procurement Resource is an invaluable partner for businesses seeking comprehensive market research and strategic insights across a spectrum of industries. With a repository of over 500 chemicals, commodities, and utilities, updated regularly, they offer a cost-effective solution for diverse procurement needs. Their team of seasoned analysts conducts thorough research, delivering clients with up-to-date market reports, cost models, price analysis, and category insights.
By tracking prices and production costs across various goods and commodities, Procurement Resource ensures clients receive the latest and most reliable data. Collaborating with procurement teams across industries, they provide real-time facts and pioneering practices to streamline procurement processes and enable informed decision-making. Procurement Resource empowers clients to navigate complex supply chains, understand industry trends, and develop strategies for sustainable growth.
Contact Us:
Company Name: Procurement Resource
Contact Person: Amanda Williams
Email: sales@procurementresource.com
Toll-Free Number: USA Canada – Phone no: +1 307 363 1045 | UK – Phone no: +44 7537 132103 | Asia-Pacific (APAC) – Phone no: +91 1203185500
Address: 30 North Gould Street, Sheridan, WY 82801, USA



