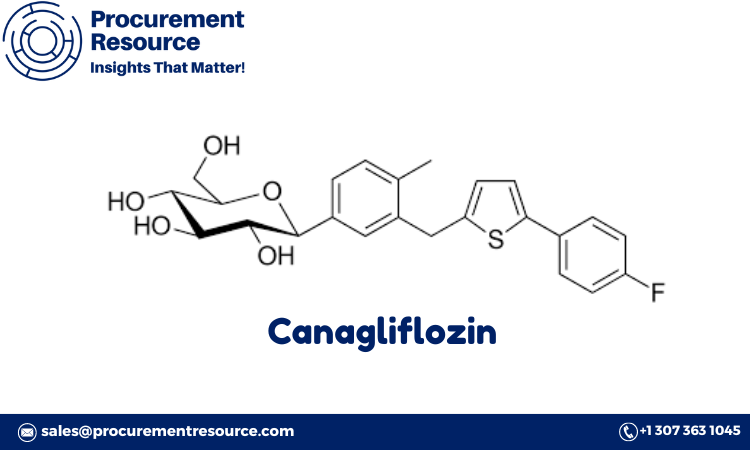
Canagliflozin is a sodium-glucose co-transporter 2 (SGLT2) inhibitor used to manage type 2 diabetes. This innovative pharmaceutical compound helps regulate blood glucose levels by preventing glucose reabsorption in the kidneys, offering an effective treatment option for diabetic patients. This report provides a comprehensive overview of the Canagliflozin Production Process with Cost Analysis, covering procurement resource assessment, market drivers, raw material needs, and production costs. For pharmaceutical companies involved in diabetes treatment development and manufacturing, this guide offers essential information on the canagliflozin production process and key factors influencing its cost structure.
Request Free Sample – https://www.procurementresource.com/production-cost-report-store/zinc-lactate/request-sample
Procurement Resource Assessment: Canagliflozin Production Process
The production of canagliflozin involves several critical resources, including specialized raw materials, advanced equipment, and trained personnel. Effective procurement is crucial to ensure consistent quality, cost efficiency, and regulatory compliance throughout the production process.
Key components of procurement resource assessment include:
- Raw Material Sourcing: Canagliflozin synthesis relies on specific chemical compounds and precursors, including specialized reagents and active pharmaceutical ingredients (APIs). Securing high-quality pharmaceutical-grade materials is essential for maintaining consistent production yields and product purity. Establishing long-term partnerships with reliable suppliers ensures stable access to these critical raw materials.
- Production Facility and Equipment: Canagliflozin production requires specialized equipment, such as chemical reactors, filtration units, and purification systems. Facilities must meet stringent Good Manufacturing Practices (GMP) and comply with pharmaceutical regulations to ensure quality and safety. Many pharmaceutical manufacturers invest in automated systems to optimize production efficiency and maintain rigorous quality control standards.
- Labor and Skilled Personnel: Producing canagliflozin involves complex chemical synthesis processes that require skilled personnel with expertise in organic chemistry and pharmaceutical manufacturing. Employing trained staff for process monitoring, quality assurance, and compliance with regulatory standards is essential for efficient and reliable production.
- Energy and Utilities: The production process is energy-intensive, particularly during synthesis, crystallization, and drying stages. Reliable access to electricity, steam, and purified water is critical to maintain controlled production conditions and ensure high-quality output.
A comprehensive procurement resource assessment allows producers to secure the necessary inputs for efficient canagliflozin production, optimize costs, and maintain compliance with regulatory requirements, ultimately supporting the consistent delivery of this essential diabetes medication.
Understanding Canagliflozin and Its Applications
Canagliflozin is a sodium-glucose co-transporter 2 (SGLT2) inhibitor that helps lower blood glucose levels in patients with type 2 diabetes. By inhibiting the SGLT2 protein in the kidneys, canagliflozin reduces glucose reabsorption, leading to increased glucose excretion in the urine. This unique mechanism of action helps manage blood sugar levels and supports overall diabetes control.
Applications of canagliflozin include:
- Type 2 Diabetes Management: Canagliflozin is primarily used as a treatment for type 2 diabetes, helping to improve glycemic control. It is often prescribed in combination with other diabetes medications, such as metformin, to achieve optimal blood glucose management.
- Cardiovascular and Renal Benefits: Studies have shown that canagliflozin may offer additional benefits for patients with cardiovascular disease and diabetic nephropathy. By reducing blood pressure and proteinuria, it helps protect the kidneys and lower the risk of heart disease in diabetic patients.
- Weight Management and Blood Pressure Control: As an added benefit, canagliflozin supports weight management and blood pressure reduction, which are common issues in diabetic patients. Its ability to promote glucose excretion contributes to calorie loss, which aids in weight reduction and improved metabolic health.
Due to its broad therapeutic effects, canagliflozin is a valuable medication for type 2 diabetes management, offering benefits beyond blood glucose control and improving patients’ overall health outcomes.
Market Drivers Influencing Canagliflozin Production
The production and demand for canagliflozin are influenced by several market drivers, including the prevalence of diabetes, advancements in diabetes treatment, and regulatory considerations. Understanding these drivers is essential for pharmaceutical manufacturers and investors to make informed decisions about their operations and strategies.
- Rising Prevalence of Type 2 Diabetes: The global prevalence of type 2 diabetes continues to rise, driven by factors such as aging populations, sedentary lifestyles, and poor dietary habits. As the number of diabetes patients increases, so does the demand for effective diabetes medications like canagliflozin.
- Advancements in Diabetes Treatment: Canagliflozin is part of a newer class of diabetes medications, the SGLT2 inhibitors, which offer unique benefits compared to traditional treatments. The demand for innovative diabetes management solutions that address multiple health concerns, such as weight and cardiovascular health, supports the growth of canagliflozin production.
- Supportive Healthcare Policies and Reimbursement Programs: Many countries provide reimbursement and insurance coverage for diabetes medications, making canagliflozin more accessible to patients. Additionally, government initiatives aimed at improving diabetes management support the demand for advanced treatments, encouraging production and distribution.
- Ongoing Research and Expanded Indications: Continuous research on canagliflozin’s potential benefits for cardiovascular and renal health has expanded its indications. This growth in therapeutic applications creates opportunities for increased production and distribution to serve a broader patient population.
- Stringent Regulatory Requirements and Quality Standards: Canagliflozin production must adhere to rigorous regulatory standards for pharmaceutical products, ensuring safety, quality, and efficacy. Compliance with Good Manufacturing Practices (GMP) and global regulatory standards builds trust with healthcare providers and patients, supporting demand for this essential medication.
By understanding these market drivers, canagliflozin producers can align their strategies to meet growing healthcare needs, capitalize on advancements in diabetes treatment, and maintain compliance with regulatory standards.
Raw Materials Requirements for Canagliflozin Production
The production of canagliflozin requires specific raw materials, including active pharmaceutical ingredients (APIs), chemical reagents, and solvents used in the synthesis process. Key raw material requirements include:
- Active Pharmaceutical Ingredients (APIs): The synthesis of canagliflozin involves key APIs and intermediate compounds, such as glucose derivatives and benzene ring compounds. Pharmaceutical-grade materials are essential to ensure consistent production yields and maintain product purity.
- Chemical Reagents and Solvents: The chemical synthesis process requires various reagents and solvents, including acetone, ethanol, and organic acids, to facilitate reactions and purify the final product. Reliable sourcing of high-quality solvents and reagents is necessary to optimize production efficiency and product quality.
- Purification and Crystallization Agents: After synthesis, canagliflozin undergoes purification and crystallization to remove impurities. Common agents for these steps include acid and base solutions, as well as activated carbon or silica for filtration. These materials must meet stringent quality standards for pharmaceutical applications.
- Water and Sterilization Agents: Purified water is used throughout the production process, particularly in washing, dissolving, and formulating canagliflozin for tablets or injectable forms. Sterilization agents, such as steam or ethylene oxide, are used to maintain sterile conditions and prevent contamination during production.
By managing these raw material requirements effectively, producers can optimize the synthesis and purification processes, control costs, and ensure the quality of canagliflozin for pharmaceutical applications.
Costs and Key Process Information
The cost of producing canagliflozin depends on several factors, including raw material prices, labor expenses, equipment investment, and energy consumption. Additionally, production costs vary based on the scale of production, facility location, and technological advancements in synthesis and purification processes.
- Raw Material Costs: The cost of APIs, chemical reagents, and purification agents represents a significant portion of canagliflozin production expenses. Prices for these materials can fluctuate based on supplier agreements, quality requirements, and market conditions. Establishing long-term contracts with reliable suppliers helps stabilize costs.
- Energy Costs: Canagliflozin production is energy-intensive, particularly for synthesis, crystallization, and drying. Energy expenses for electricity and steam significantly impact total production costs. Many facilities invest in energy-efficient equipment to reduce operational expenses and support sustainability initiatives.
- Labor and Compliance Costs: Skilled chemists, technicians, and quality control specialists are essential for producing canagliflozin. Labor costs depend on the location and production scale, while compliance with regulatory standards contributes to operational expenses. Automation and digital monitoring systems can help reduce labor costs and improve process efficiency.
- Equipment and Maintenance: The initial investment in reactors, crystallizers, and filtration systems is substantial. Ongoing maintenance is necessary to ensure operational efficiency and prevent contamination. Producers must also budget for periodic equipment upgrades or replacements to meet evolving standards.
- Regulatory Compliance and Quality Assurance: Canagliflozin production must comply with stringent quality and regulatory standards, particularly for pharmaceutical applications. Adherence to Good Manufacturing Practices (GMP) and quality assurance protocols ensures product safety and efficacy. These requirements contribute to production costs but are essential for regulatory approval and patient safety.
Looking for an Exhaustive and Personalized Report?
For businesses seeking a thorough understanding of the canagliflozin production process with cost analysis, a personalized and exhaustive report can provide valuable insights. Such a report includes detailed cost breakdowns, profitability forecasts, and customized recommendations for optimizing production, enhancing efficiency, and expanding market reach. Whether you aim to scale operations, explore new market opportunities, or improve process efficiency, an industry-specific report can substantiate your business strategies with data-driven analysis.
With comprehensive market data, cost projections, and strategic guidance, a customized report on canagliflozin production can empower you to make informed decisions that drive growth, sustainability, and profitability in your business.
About Us:
Procurement Resource is an invaluable partner for businesses seeking comprehensive market research and strategic insights across a spectrum of industries. With a repository of over 500 chemicals, commodities, and utilities, updated regularly, they offer a cost-effective solution for diverse procurement needs. Their team of seasoned analysts conducts thorough research, delivering clients with up-to-date market reports, cost models, price analysis, and category insights.
By tracking prices and production costs across various goods and commodities, Procurement Resource ensures clients receive the latest and most reliable data. Collaborating with procurement teams across industries, they provide real-time facts and pioneering practices to streamline procurement processes and enable informed decision-making. Procurement Resource empowers clients to navigate complex supply chains, understand industry trends, and develop strategies for sustainable growth.
Contact Us:
Company Name: Procurement Resource
Contact Person: Amanda Williams
Email: sales@procurementresource.com
Toll-Free Number: USA Canada – Phone no: +1 307 363 1045 | UK – Phone no: +44 7537 132103 | Asia-Pacific (APAC) – Phone no: +91 1203185500
Address: 30 North Gould Street, Sheridan, WY 82801, USA



